In the world of cell culture research, maintaining uncontaminated cell lines is essential to achieving accurate, reliable results. One of the most common yet insidious contaminants is mycoplasma—a small, cell-wall-deficient bacterium that can infiltrate cultures, disrupt cell physiology, and invalidate experimental outcomes. Addressing this, the MycAway® Mycoplasma Real-Time qPCR Detection Kit offers a fast, accurate, and user-friendly solution to identify and manage mycoplasma contamination, safeguarding the integrity of your cell cultures.
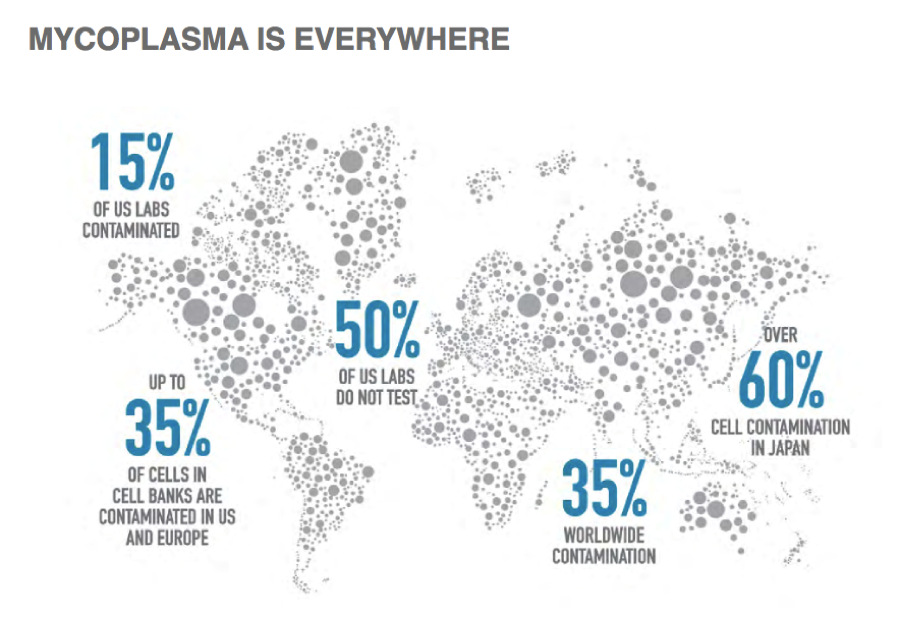
Why Mycoplasma Contamination is a Major Issue in Cell Culture
Unlike typical bacterial contaminants, mycoplasmas are challenging to detect due to their small size and lack of a cell wall. They are resistant to most antibiotics commonly used in cell culture, allowing them to persist undetected. Mycoplasma contamination poses several significant risks:
- Altered Cellular Behavior: Mycoplasmas can interfere with cellular metabolism, gene expression, and even chromosomal stability, leading to inconsistent and unreliable data.
- Loss of Experimental Validity: Contaminated cell lines may produce misleading results, affecting studies in fields like gene expression, drug discovery, and toxicology.
- Wasted Time and Resources: If contamination goes undetected, researchers may have to repeat experiments, clean equipment, and potentially replace cell lines, leading to considerable time and financial losses.
Given these challenges, routine screening for mycoplasma is essential for labs working with cell cultures, ensuring both the validity and reproducibility of experimental results.
Introducing the MycAway® Mycoplasma Real-Time qPCR Detection Kit
The MycAway® Mycoplasma Real-Time qPCR Detection Kit provides a rapid, precise solution for detecting mycoplasma contamination in cell cultures. Designed to deliver results in less than two hours, MycAway® combines the sensitivity and specificity of quantitative PCR (qPCR) with a user-friendly format. Here’s how MycAway® excels:
- High Sensitivity: With a detection limit as low as 10 CFU/mL, MycAway® ensures even minimal mycoplasma contamination can be identified. This high sensitivity allows for early intervention before contamination becomes widespread.
- Quick Turnaround: Unlike traditional methods, which can take days, MycAway® provides results in under two hours, enabling rapid decision-making and preventing delays in your research workflow.
- Broad Species Detection: MycAway® targets multiple species of mycoplasma, including M. hyorhinis, M. arginini, and M. orale—common contaminants in cell culture—ensuring that your detection coverage is comprehensive.
- All-Inclusive Kit: The MycAway® kit contains everything needed for mycoplasma detection, including primers, probes, and both positive and negative controls, simplifying the process and providing assurance in the accuracy of your results.
- Ease of Use: Suitable for both novice and experienced researchers, MycAway® fits seamlessly into standard lab workflows, making it a practical choice for routine mycoplasma screening.
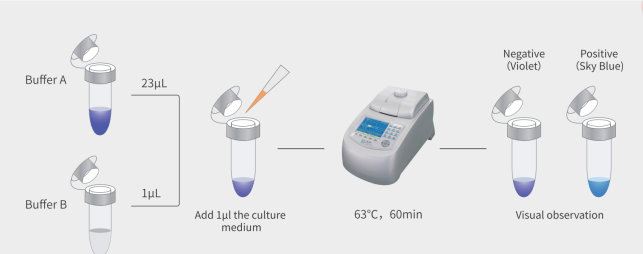
How the MycAway® Detection Kit Works
The MycAway® Mycoplasma Real-Time qPCR Detection Kit leverages qPCR technology, a powerful method that combines the specificity of PCR with real-time detection. Here’s a breakdown of the detection process:
- Sample Collection: Researchers collect samples from cell culture supernatants or media. The MycAway® protocol requires minimal handling, reducing the risk of cross-contamination and ensuring accurate detection.
- qPCR Amplification: The qPCR reaction specifically amplifies mycoplasma DNA using primers and probes included in the MycAway® kit. As amplification occurs, a fluorescent signal is generated, with the intensity of fluorescence directly correlating with the amount of mycoplasma DNA present.
- Real-Time Results: Fluorescence data is measured in real-time, providing an immediate indication of mycoplasma contamination. Results can be interpreted with ease, with clear thresholds to differentiate contaminated and uncontaminated samples.
Why Choose the MycAway® Mycoplasma Real-Time qPCR Detection Kit?
The MycAway® Mycoplasma Real-Time qPCR Detection Kit offers a practical, reliable, and efficient approach to maintaining uncontaminated cell cultures. Here’s why MycAway® stands out:
- GMP-Grade Manufacturing: Manufactured under Good Manufacturing Practice (GMP) standards, MycAway® meets stringent quality control requirements, ensuring the reliability and consistency of each kit.
- Versatile Compatibility: MycAway® is compatible with various qPCR platforms, including those from Bio-Rad, ABI, and Thermo, allowing it to integrate smoothly with existing lab equipment.
- Multi-Species Coverage: By detecting multiple mycoplasma species, the kit provides broad contamination coverage, making it an effective choice for labs handling a variety of cell types and culture conditions.
- Control Components: Positive and negative controls included in each kit help validate results, reducing the risk of false positives or negatives.
Implementing MycAway® in Your Lab Routine
To ensure the highest standards of culture integrity, it’s recommended to screen cell cultures with MycAway® at key stages:
- Upon Receiving New Cell Lines: Before integrating any new cell line into your existing work, a quick mycoplasma screen can prevent the spread of contamination.
- Post-Thaw: Thawed cells should be tested to confirm they’re contamination-free before proceeding with any experiments.
- Regular Screening: Routine screening every few weeks helps maintain a contamination-free lab, especially in long-term culture experiments.
By adopting a routine mycoplasma detection protocol with MycAway®, labs can avoid the costly impacts of contamination and protect the validity of their research.
Final Thoughts
Mycoplasma contamination poses a serious risk to cell culture research, but with the MycAway® Mycoplasma Real-Time qPCR Detection Kit, researchers now have a powerful tool to safeguard their cultures. The MycAway® kit offers high sensitivity, quick results, and ease of use, making it a must-have for any lab working with cell cultures. By investing in regular mycoplasma screening, you protect not only your cell lines but also the reliability of your research outcomes.
Preserve the integrity of your cell culture research with MycAway®—a fast, accurate, and comprehensive solution for mycoplasma detection.