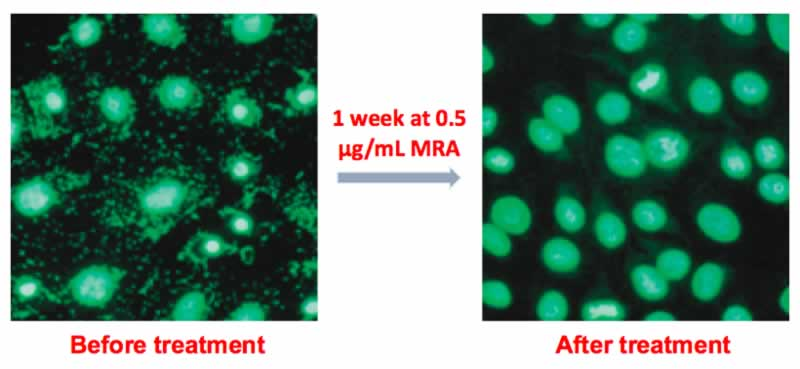
In cell culture research, maintaining uncontaminated cultures is critical for reliable results and experimental success. One of the most common, yet often undetected contaminants, is mycoplasma. These bacteria can infiltrate cultures, alter cellular physiology, and invalidate research findings. To protect your cell cultures and ensure the integrity of your work, the MycAway® Mycoplasma Real-Time qPCR Detection Kit offers a highly accurate, efficient, and easy-to-use solution for mycoplasma detection.
The Challenge of Mycoplasma Contamination
Mycoplasmas are small, cell wall-deficient bacteria that are challenging to detect due to their small size and atypical structure. These contaminants are resistant to many antibiotics used in cell culture and can spread quickly through laboratory environments. Mycoplasma contamination can cause significant issues, including:
- Altered Cell Physiology: Mycoplasma can change cell metabolism, growth rates, and gene expression, leading to unreliable experimental data.
- Loss of Experimental Validity: Contaminated cell lines often yield inaccurate results, impacting everything from gene expression studies to drug testing.
- Time and Resource Loss: Undetected contamination can mean lost time, money, and resources, as experiments must be redone, and equipment cleaned or replaced.
Due to these impacts, mycoplasma contamination can undermine months of work and compromise experimental validity. Regular screening and detection are essential for early identification and elimination.
MycAway® Mycoplasma Real-Time qPCR Detection Kit: A Reliable Solution
The MycAway® Mycoplasma Real-Time qPCR Detection Kit provides a powerful tool for detecting mycoplasma contamination in cell cultures. Here’s what sets it apart:
- High Sensitivity and Specificity
MycAway® uses advanced qPCR technology to detect even low levels of mycoplasma contamination. The kit targets multiple mycoplasma species, ensuring broad coverage and minimizing false negatives. With a detection limit as low as 10 CFU/mL, the kit identifies contamination early, enabling swift intervention. - Quick and Accurate Results
Traditional mycoplasma detection methods, such as agar and broth cultures, can take days or even weeks. The MycAway® qPCR kit provides results in under two hours, allowing researchers to quickly determine contamination status and take action if needed. - Ease of Use
The MycAway® kit is designed for straightforward application, making it accessible for both novice and experienced researchers. It includes all necessary components, from primers and probes to positive and negative controls, streamlining the workflow and ensuring consistency. - Cost-Effective Solution
Preventing contamination with regular screening using MycAway® can save time and money in the long run by reducing the need for repeating experiments or replacing valuable cell lines. This proactive approach is far more economical than dealing with the repercussions of a full-blown contamination.
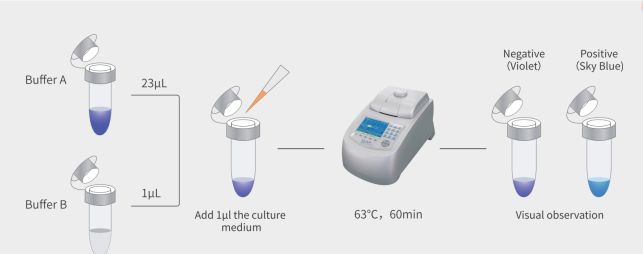
How the MycAway® Mycoplasma Real-Time qPCR Detection Kit Works
The MycAway® detection kit leverages the precision of real-time quantitative PCR (qPCR) technology. Here’s an overview of the process:
- Sample Preparation
Samples are collected directly from cell culture supernatants or culture media. MycAway® minimizes handling time, reducing the risk of contamination during the preparation stage. - qPCR Amplification
The kit uses specific primers and probes that bind to mycoplasma DNA, enabling selective amplification of these bacterial sequences. During amplification, a fluorescent signal is generated, which increases proportionally with the amount of mycoplasma DNA present. - Result Interpretation
The qPCR instrument quantifies fluorescence in real-time, providing an immediate indication of contamination levels. Results are easy to interpret, with a clear threshold separating contaminated and uncontaminated samples. Positive and negative controls included in the kit ensure that results are accurate and reliable.
Why Choose MycAway® for Mycoplasma Detection?
- GMP-Grade Quality: The MycAway® kit is manufactured in a GMP-compliant facility, ensuring high standards of quality and consistency. It’s designed to meet rigorous laboratory and clinical requirements, making it a trusted tool for contamination prevention.
- Compatibility with Various qPCR Platforms: MycAway® works with a wide range of qPCR systems, including Bio-Rad, ABI, and Thermo platforms, giving laboratories the flexibility to integrate it into their existing equipment.
- Multi-Species Detection: The kit is optimized to detect multiple species of mycoplasma that commonly contaminate cell cultures, including M. orale, M. arginini, and M. hyorhinis, among others. This broad detection capability is essential for comprehensive contamination screening.
- Comprehensive Controls for Accuracy: The inclusion of positive and negative controls allows researchers to confirm the accuracy of their results, reducing the risk of both false positives and false negatives.
Incorporating MycAway® into Your Lab Routine
Regular screening with MycAway® is a simple yet powerful way to prevent contamination. For best results, it’s recommended to screen cell cultures at regular intervals, ideally:
- Before introducing new cell lines into the laboratory.
- After thawing frozen cell stocks to ensure they’re free from contamination.
- Regularly throughout long-term culture experiments to catch contamination early.
Final Thoughts
Mycoplasma contamination poses a serious risk to cell culture research, but with the MycAway® Mycoplasma Real-Time qPCR Detection Kit, researchers can proactively protect their work. Its high sensitivity, ease of use, and rapid turnaround time make it an invaluable tool for any laboratory working with cell cultures. By incorporating MycAway® into your lab routine, you safeguard your research and ensure that your findings are based on pure, uncontaminated cell lines.
Protect your research and maintain the highest standards of cell culture integrity with the MycAway® Mycoplasma Real-Time qPCR Detection Kit.