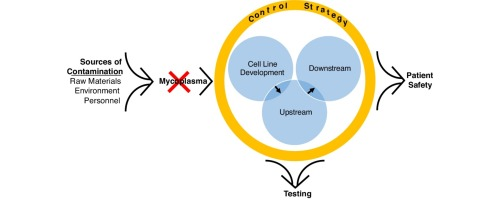
The MycAway® Mycoplasma Real-Time qPCR Detection Kit represents an essential tool for any lab seeking reliable and efficient mycoplasma detection in cell cultures. Contamination by mycoplasma can compromise research results, affect cellular physiology, and ultimately lead to costly setbacks. With the MycAway® kit, researchers can confidently detect mycoplasma contamination early, ensuring the integrity of their cell cultures and the accuracy of their experimental data.
Why Mycoplasma Detection Matters
Mycoplasma contamination is a significant issue in cell culture laboratories, with studies suggesting that between 10% and 30% of all cell lines in research labs worldwide may be contaminated. Unlike other bacterial infections, mycoplasma contamination is often invisible to the naked eye. It doesn’t typically cause visible turbidity in the medium, so standard visual checks are ineffective. However, even at low levels, mycoplasma can alter cell physiology, affecting gene expression, cell proliferation, and even metabolic rates. This silent contamination can lead to erroneous results and misinterpretation of experimental data.
Features of the MycAway® Mycoplasma Detection Kit
The MycAway® Mycoplasma Real-Time qPCR Detection Kit is designed to meet the rigorous demands of scientific research and industry applications. Here are some of the key features that set this kit apart:
- Comprehensive Detection Capability: This kit is capable of detecting a wide range of mycoplasma species commonly found in contaminated cell cultures. It includes controls and reagents tailored for detecting mycoplasma DNA with high sensitivity, ensuring no contamination goes unnoticed.
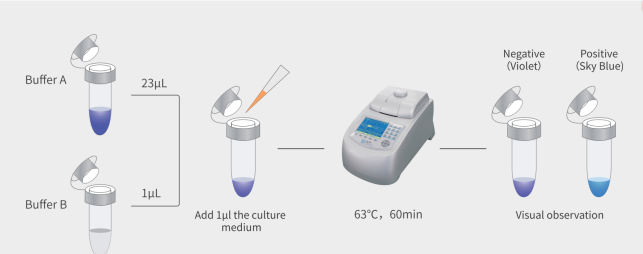
- Fast and Accurate Results: Utilizing real-time quantitative PCR (qPCR) technology, the MycAway® kit provides results quickly—ideal for labs that require timely answers to maintain experimental timelines. With this kit, mycoplasma detection is not only faster but also more accurate compared to traditional methods like culture assays, which can take weeks.
- High Sensitivity and Specificity: The MycAway® qPCR kit boasts an extremely low detection limit, capable of detecting as few as 10 CFU/mL, which meets pharmacopeia standards. This level of sensitivity ensures that even minimal contamination can be identified and addressed promptly.
- User-Friendly Protocol: The kit is designed with simplicity in mind, enabling even less experienced technicians to conduct tests accurately. The straightforward protocol minimizes the chances of user error, making it accessible for labs of all sizes and experience levels.
- Compatibility with Standard Equipment: The MycAway® kit is compatible with various qPCR instruments commonly used in laboratories, including models from Thermo, Roche, Bio-Rad, and more. This broad compatibility makes it an easy addition to any lab setup.
Applications of the MycAway® Mycoplasma Detection Kit
The MycAway® kit is versatile and suitable for a variety of applications, including:
- Routine Screening: Regular testing of cell lines to ensure they remain free from contamination, which is critical for labs maintaining long-term cultures or working on sensitive projects.
- Validation of Cell Banks: Before cell lines are stored or shared, validating them for mycoplasma-free status ensures that valuable cell lines are not compromised.
- Quality Control in Biopharma: For biopharmaceutical production, ensuring the purity of cell cultures used in drug production is mandatory. The MycAway® kit serves as an essential part of quality control protocols in pharmaceutical manufacturing.
Technical Specifications
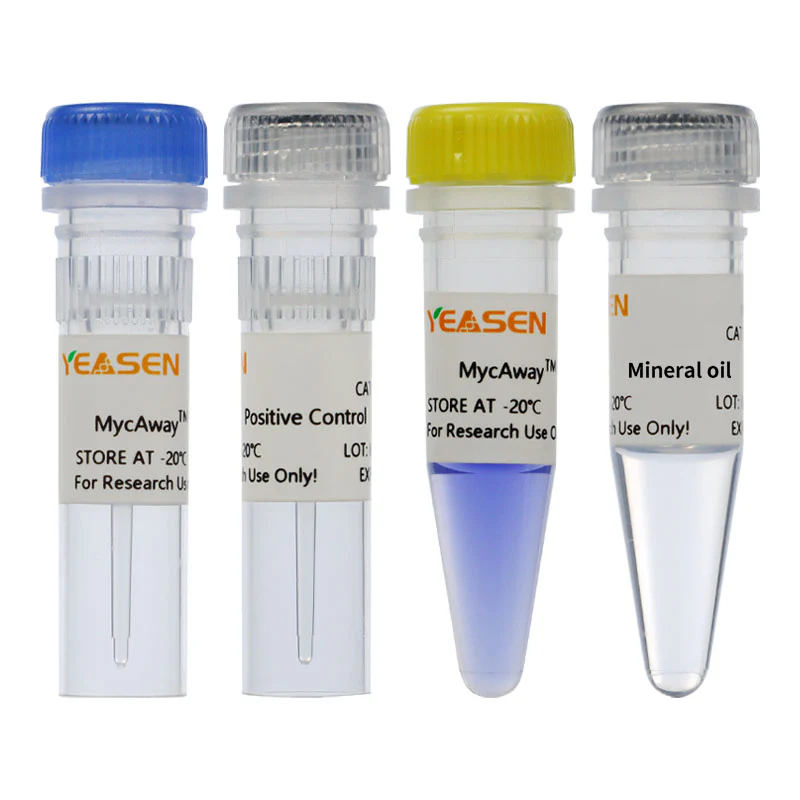
The MycAway® kit includes all necessary reagents for mycoplasma detection, including MyqPCR Reaction Buffer, primers and probes specific for mycoplasma, and both positive and internal controls. The kit also comes with DEPC-treated water for optimal reaction conditions. By meeting stringent standards for pharmaceutical applications, the MycAway® kit ensures that results are reliable and reproducible.
Additionally, the kit includes a magnetic bead-based pretreatment option, allowing labs to use it with a variety of sample types. Its robust design meets the pharmacopoeia requirement of 10 CFU/mL, making it an invaluable tool for any lab where precision and accuracy are paramount.
Conclusion: Protecting Your Research with MycAway®
The MycAway® Mycoplasma Real-Time qPCR Detection Kit empowers researchers and production facilities with a reliable, fast, and sensitive method for detecting mycoplasma contamination. By ensuring that cell cultures remain uncontaminated, the MycAway® kit protects the validity of your data, the success of your projects, and ultimately, the credibility of your laboratory. Whether in academic research, clinical labs, or biopharmaceutical manufacturing, this kit is a powerful ally in the fight against unseen contaminants.
For any laboratory dealing with cell culture, safeguarding research from the silent threat of mycoplasma contamination is non-negotiable. With MycAway®, you can conduct your research with confidence, knowing that your cell lines are protected and your results are accurate.